What is conventional TSA separation and concentration technology?
To understand the innovation of Wet-TSA adsorption concentration technology, it is necessary to understand it from the conventional TSA (Thermal Swing Adsorption) method. The TSA method is a method in which gas substances are adsorbed to an adsorbent, and then the concentrated gas substances are desorbed and collected by heating.
For example
1. A desiccant dehumidifier is a method of producing dry air by adsorbing and removing water vapor from the air using a desiccant (moisture absorbent). The principle is that the adsorbed water vapor is heated and desorbed to regenerate it, and the system returns to the next dehumidification operation for continuous dehumidification.
2. A VOC (Volatile Organic Compound) concentration device adsorbs and removes VOCs evaporated during painting, printing, etc., onto a VOC adsorbent before exhausting the air, purifying it, and exhausting it. The adsorbed VOCs can be heated, for example, to a concentration 10 times higher, and then desorbed and collected, making it one-tenth as efficient as combustion detoxification.
Disadvantages of conventional TSA adsorption separation methods
Water vapor exists everywhere on earth. In the case of dehumidifiers, water vapor is adsorbed and removed, so it is not a problem at all, but it is troublesome when adsorbing and removing gaseous substances other than water vapor. When adsorbing gaseous substances other than water vapor, water vapor is also adsorbed, inhibiting the adsorption of the gaseous substances. During desorption, energy is wasted in desorbing the adsorbed water vapor, significantly inhibiting performance. For this reason, researchers have made efforts to study hydrophobic (water vapor-avoiding) adsorbents and research on removing water vapor in advance.
Another drawback of the TSA method that I have focused on is the generation of adsorption heat when adsorbing gaseous substances. The adsorption heat of gaseous substances corresponds to the debt of latent heat when the substance evaporates from a liquid, and is thermodynamically bound to occur. This adsorption heat raises the temperature of the adsorbent and the treated gas, significantly reducing the adsorption power. This is unavoidable in the TSA method, which uses temperature difference for adsorption and desorption, and it is particularly impossible to separate high-concentration gases or recover them at high concentrations.
Principles of Wet-TSA and reasons for its superiority
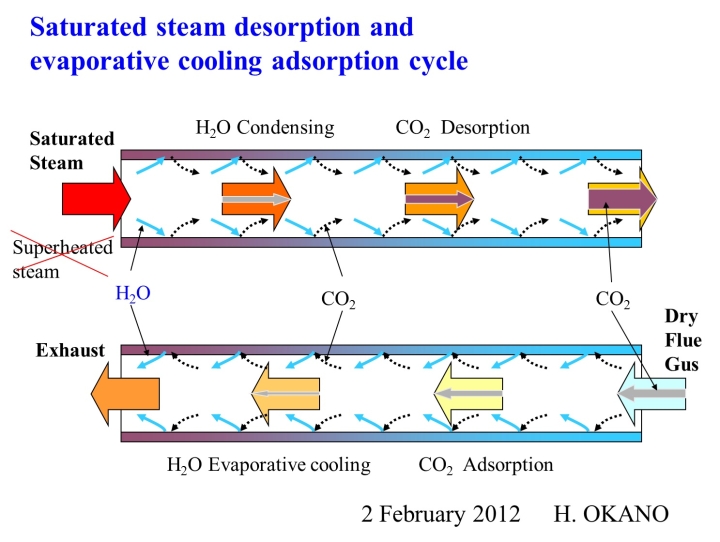
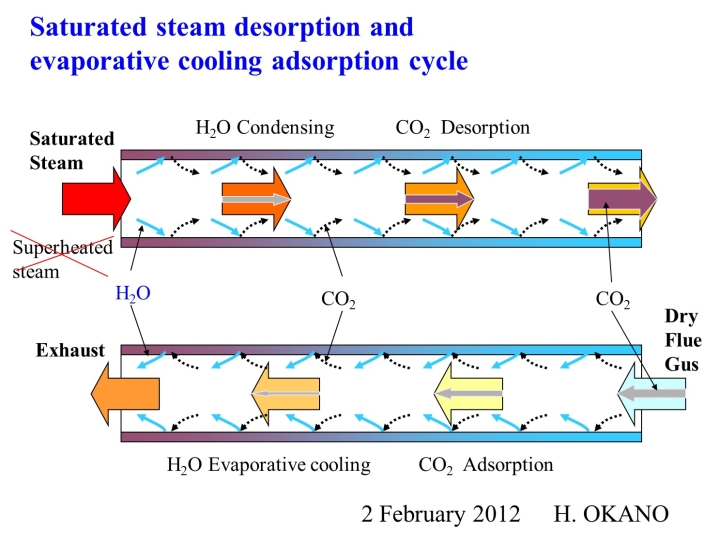
The Wet-TSA method is based on the opposite idea to the conventional TSA method, which aims to avoid the influence of water vapor as much as possible. It is a method of adsorbing and desorbing CO2 in a constantly wet state, and the temperature change caused by the transition between water and water vapor does not hinder the adsorption and desorption performance, but rather acts to assist it, dramatically improving the adsorption and concentration performance.
The principle of the Wet-TSA method is that saturated steam is introduced into the adsorbent that has adsorbed gas, and the adsorbed CO2 gas is desorbed using the heat of condensation of the saturated steam. The heat capacity per volume of saturated steam is tens to hundreds of times that of dry air, so gas can be desorbed with a small amount of saturated steam, and the desorbed gas can be concentrated to nearly 100% concentration and extracted.
In the conventional TSA method, air is heated and used as the desorption gas, so air is always mixed in with the desorbed gas, making it impossible to recover a high concentration.
When adsorbing gas with the Wet-TSA method, the water vapor that condenses during desorption does a good job. When the condensed water evaporates as the treated gas passes through, the adsorbent is cooled by the evaporative cooling effect, improving the adsorption performance. At the same time, the heat of adsorption of the gas is also removed by the evaporative cooling effect, so the air temperature of the treated gas hardly rises at all, which is why the Wet-TSA method is able to achieve dramatically high performance.
Difference from conventional superheated steam regeneration
Steam regeneration, in which steam is heated to over 100°C for desorption, has been around for some time. VOC recovery using activated carbon adsorbents is also known. This method involves cooling the mixed gas desorbed with steam, condensing the steam, and recovering the VOCs and condensed water. Since no air is introduced, this method has the advantage of avoiding activated carbon fires and oxidation deterioration of VOCs. If activated carbon becomes wet with condensed water, it will not adsorb VOCs, so the overheating temperature must be increased to prevent condensation, or a cooling and drying process may be provided after desorption.
The Wet-TSA method has the major difference and advantage of using saturated steam at around 100°C for desorption. In other words, the moment saturated steam comes into contact with the adsorbent, it generates condensation heat and desorbs CO2. The amount of condensed water is similar to the amount of desorbed CO2 and the latent heat (condensation and evaporation or adsorption and desorption), so more condensation occurs on adsorption sites with a large amount of desorption, and less condensed water occurs on adsorption sites with a small amount of desorption. Therefore, there is no excess condensed water that causes flooding.
What happens if superheated steam becomes saturated steam after being introduced into the rotor?
No condensed water will be generated in the section desorbed with superheated steam, and the evaporative cooling effect will not occur in the adsorption process, resulting in a decrease in adsorption performance.
Further advantages of the Wet-TSA method
There are various CO2 adsorbents, but amine-based adsorbents are advantageous for desorption at low temperatures to around 100°C. However, amine-based adsorbents have the disadvantage of being vulnerable to thermal oxidative degradation. For this reason, various research institutes and researchers are conducting research to improve performance and durability. In addition, adsorption and concentration systems that do not undergo thermal oxidative degradation are being researched and patent applications are being filed.
G.V.Lab.'s Wet-TSA method also incorporates the prevention of thermal oxidative degradation.
1. Because it is a Wet-TSA method
① The adsorbent does not come into contact with oxygen by desorbing with saturated steam that does not contain oxygen.
② Even if the adsorbent is moved to the adsorption zone at a high temperature after desorption, the surface of the adsorbent is covered with condensed water, and it is quickly cooled by the evaporative cooling effect of the condensed water, so oxidative degradation is avoided.
③ The durability of amine-based adsorbents may be improved by always maintaining a hydrated state
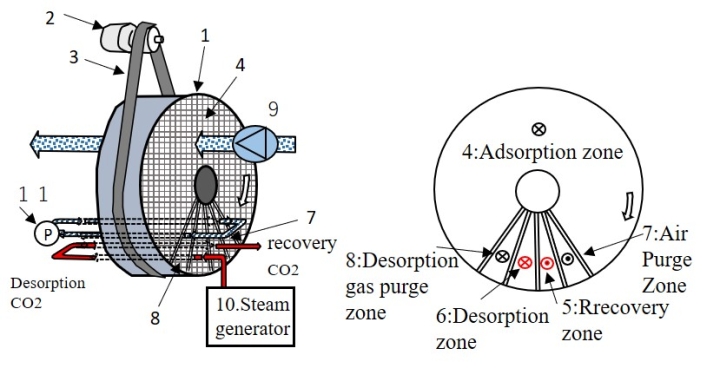
2. Revolutionary G.V.Lab. Wet-TSA patent flow
①Before the rotor moves to the desorption zone where the temperature is the highest, the mixture of desorbed CO2 and water vapor is passed through the capture zone to be captured, preventing the introduction of oxygen into the desorption zone and preventing thermal oxidation deterioration.
②A circulation purge zone, which combines a gas purge zone and an air purge zone, is provided before and after the desorption zone and capture zone to exchange air for CO2 gas. This circulation purge allows the CO2 brought from the desorption zone to the adsorption zone by the rotation of the rotor to be captured in the gas purge zone, and the air brought in from the adsorption zone is purged by introducing it into the air purge zone, which is expected to reduce the transfer of oxygen to the capture zone and at the same time reduce the loss of captured CO2 gas.